Kossel-Lewis Approach to Chemical Bonding
Kossel-Lewis Approach to Chemical Bonding: Overview
This topic covers concepts such as Atom, Lewis Symbols of Elements, Significance of Lewis Symbols, Lewis Dot Structure, Steps for writing Lewis Dot Structure, Lewis Structure for Simple Molecules, Covalent Bond, etc.
Important Questions on Kossel-Lewis Approach to Chemical Bonding
What are the formal charges on and in the Lewis (electron dot) structure of the phosphate oxyanion represented in the figure?
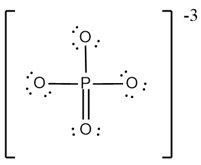
Give 2 drawbacks of Kossel's Postulate.
Mention few applications of formal charges?
_____ is a factor based on a pure covalent view of bonding in which electron pairs are shared equally by neighbouring atoms.
Formal charges do not indicate real charge separation within the molecule.
How will you calculate the valency of potassium?
Define the group valency.
List some odd electron molecules.
Identify odd-electron molecules from the following:
Which of the following oxides is an odd-electron molecule.
Which of the following bonds can be present in a chemical compound?
Hydrogen gas is a chemical compound.
Which of the following is a Chemical Compound?
Which of the following is not a Chemical Compound?
The Xenon difluoride can act as _____.
Mark the following statement as true or false.
Helium can also form its compounds.Why noble gases do not form compounds except krypton, xenon, and radon?
Which of the following noble gas compound has hybridisation?
What are the noble gases which form compounds?
Bonding in this molecule can be understood to involve coordinate bonding.
